Explain How 4p Orbitals Are Different From 3p Orbitals.
The atomic orbitals are of different shapes where the s orbital has spherical shape the p orbital has a dumbbell shape and the d orbital has a double dumbbell shape. Factors affecting the Orbital Energy.
Why Are The Shapes Of 2p And 3p Orbitals Different In One Picture I Saw Two More Small Lobes Between Two Big Lobes Here Is The Picture Quora
For all those orbitals that belong to the same subshell it is the same and those orbitals that are with the same energy are stated as degenerate orbitals.

. Advertisement Answer 0 06gavinlim. 3d orbitals are also comparable to those of 4s and 4p orbitals. What do s orbitals look like.
There are two types of node. By the Aufbau principle 3p will be filled first before 4p. The number of angular nodes is always equal to the orbital angular momentum quantum number l.
In principle energy level n4 what type of sublevel is there. The 2s orbital would be the same shape as the 1s orbital but would be larger in size and the 3p orbital would have the same shape as the 2p orbitals bout would be larger in size. 4p orbitals are larger in size than 5p orbitals and contain additional nodes.
Each 2p orbital has two lobes. At the fourth and higher levels there are seven f orbitals in addition to the 4s 4p and 4d orbitalsCounting the 4s 4p and 4d orbitals this makes a total of 16 orbitals in the fourth level. How would the 2s and 3p orbitals differ from the 1s and 2p orbitals.
3d has 2 planar nodes giving it the familiar 4-lobed shape. S p d and f orbitals are available at all higher energy levels as well. How many orbitals are in 4p.
Difference Between Orbitals and Sublevels Sublevel A sublevel is a division of principle energy levels. The p sublevels are named 2p 3p and 4p since the p sublevel appears only starting the 2nd level. Those were very useful at times when computing power was inadequate but now it is ridiculously easy to do very accurate Full.
By that I mean it is a handwavy rule with little relevance to the modern world. All p orbitals have a characteristic dumbbell shape with a nodal plane perpendicular to the orbital axis. The 2p orbitals have more energy than the 2s orbital.
In the second electron shell n 2. 2s and three p orbitals. 4p orbitals are larger in size than 3p orbitals and contain additional nodes.
O 3p orbitals are larger in size than 4p orbitals and contain additional nodes. In the third electron shell n 3. There is a planar node normal to the axis of the orbital so the 2px orbital has a yz nodal plane for instance.
Lets have a closer look at the. Atomic orbitals describe the most likely location of the electrons that will be found around the nucleus of an atom. Now come towards your question as you ask about difference between 2p and 3p orbital the answer is that 3p orbital has same structureshape that of 2p orbital has but larger in size and energybecause it lies in 3rd orbit of an atom similarly 4p orbitals will have same shape but higher energy and larger in size but shape will be similarsimilarly 5p orbital and.
The 2s and 2p orbitals have one node. O 5p orbitals are smaller in size than 4p orbitals and contain. What does the principal quantum number tell us.
A 3p orbital has a spherical node. 5p orbitals are larger in size than 4p orbitals and contain less nodes. 4p orbitals are larger in size than 3p orbitals and contain no nodes.
I detest rules like that. Each of the p sublevel has 3 orbitals allowing them to contain 6 electrons as each orbital may hold two. Thus all orbitals with designation 3 have 3 zones where the probability of finding an electron is zero.
Also the 2s and 3p orbitals would have more nodes. A spin of an electron B orbital shape C principal energy level D speed of an electron 2 If the spin of one electron in an orbital is clockwise what is the spin of the other electron in that. The next energy level the second energy level has four orbitals.
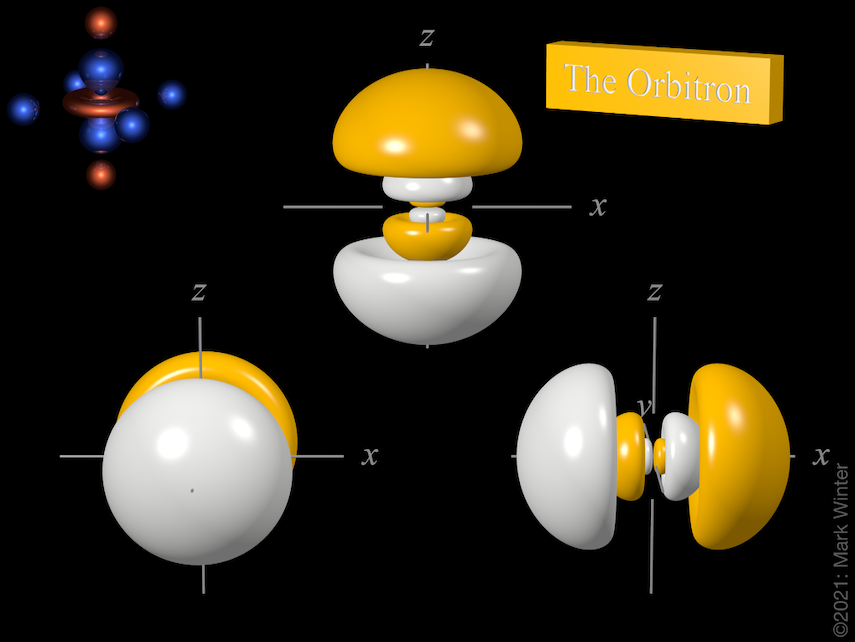
From chemwikiucdavisedu The 3p orbitals have the same general shape and are larger than 2p orbitals but they differ in the number of nodes. Explain how 4p orbitals are different from 3p orbitals. Ο Ο Ο O 5p orbitals are larger in size than 4p orbitals and contain no nodes.
4s 4p 4d 4f. 4p orbitals are larger in size than 3p orbitals and contain less nodes. However since the difference in energies of 3p and 4s orbitals are significant no.
Answer 1 of 2. You have probably noticed that the total. 3s has 2 spherical nodes the 3rd zone is infinite distance from the atom 3p has one planar and 1 spherical node.
1 The letter p in the symbol 4p3 indicates the ___. The 1s orbital is a sphere and the 2p orbital is made up of three dumbbells oriented in the x y and z direction. Item 60 Part A Explain how 5p orbitals are different from 4p orbitals.
Every unique orbital can comprise only upto two electrons. The higher p-orbitals 3p 4p 5p 6p and 7p are more complex still since they have spherical nodes as well. We see this in the 2p orbitals.
The third energy level has 3s x1. Because of this the hybridisation involving either 3s 3p and 3d or 3d 4s 4p is possible. The order of the increase in energy along the various orbitals is stated as 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f.
Theoretically speaking there are infinite numbers of sublevels but only four of them are defined which are s p d and f where s stands for sharp p for principle d for diffuse and f. This is because of the energy present on the level. The 3s 3p and 3d orbitals have two nodes etc.
They have even more complicated shapes.

Solved Explain How 4p Orbitals Are Different From 3p Chegg Com

The Gvb 3p Lobe Orbitals 3p Z 3p Z And The 3p X P Download Scientific Diagram
Comments
Post a Comment